Rhythmic IL-17 production by T cells maintains adipose de novo lipogenesis
Introduction
Circadian rhythms allow organisms to anticipate and adjust to predictable changes in the external environment such as light, feeding, and temperature. At a cellular level, the molecular clock is controlled by transcriptional activators and repressors (proteins that bind with DNA) to express/turn off certain genes. Light and feeding actually govern the circadian rhythm of adipose lipid metabolism, including lipolysis, lipogenesis, and thermogenesis. This makes sense and could have dramatic effects on metabolic health. In previous research, it has been shown that shift workers who work the night shift experience impaired lipid metabolism, leading to negative metabolic health outcomes (along with other problems too). Adipose tissue is primed to have T-cells (which are part of the adaptive immune system). One specific T-cell the study focused on is IL-17A. The reason this one is important is because IL-17A is controlled by the circadian clock, and IL-17A regulates whole-body metabolism (specifically fat metabolism). IL-17A cells are made by adipose γδ17 T cells.
Light as Regulator for IL-17A Production:
The researchers took two groups of mice and placed one group on a regular light/dark cycle and the second group on a reverse light/dark cycle. After 3 weeks, their metabolic rhythms were completely reversed. When they collected the adipose tissue, the IL-17A patterns were also reversed, showing that this T-cell is controlled by circadian rhythm, which in turn controls lipid metabolism. The researchers then separated out feeding and light control to see which impacts IL-17A more. They found that light entrains IL-17A production in adipose tissue, primarily through feeding behaviors because when eating behavior is disrupted, IL-17A is disrupted. Additionally, they found that IL-17A is a significant regulator of DNL (de novo lipogenesis), which is when the body creates fat (generally from carbohydrates).
Conclussion:
Adipose γδ17 T cells are extremely reliant on the molecular clock and produce IL-17A, which is responsible for adipose tissue circadian homeostasis, whole-body metabolism, and temperature regulation through the circadian regulation of de novo lipogenesis. One of the main roles of IL-17A is to support thermogenesis and lipid metabolism. With IL-17A present, your body supports fat storage via DNL, but without IL-17A, the body’s circadian rhythm of DNL is disrupted, impacting whole-body metabolism and temperature regulation. IL-17 is influenced by feeding, which is regulated by light. Eating during the dark phase, when DNL is more active, could lead to a tendency to store calories as fat.
Specifically in Humans:
In humans, who are diurnal, the results would be the reverse of what’s observed in nocturnal mice. IL-17A levels and de novo lipogenesis (DNL) would peak during the daytime (as opposed to nighttime in mice), when we are most active and consume most of our calories.
This means that eating in alignment with daylight hours—the human active phase—would support a natural rhythm for energy metabolism and fat storage.
Eating late at night, when we are supposed to be sleeping, could disrupt this rhythm, leading to increased fat storage and potentially affecting metabolic health. Essentially, maintaining regular meal times aligned with daylight could help optimize fat metabolism, reduce fat storage, and support overall metabolic balance.
You are probably asking why? The theory is that at night we actually mobilize fat stores for things such as temperature regulation (DNL is downregulated). If we are eating when we are supposed to be mobilizing fat stores, things can get messed up and we may not end up burning as many calories as we are supposed to be- essentially a “slow metabolism”
Diagrammatic representation of circadian whole-body metabolism by RER, showing the most active metabolic processes during the day and night, with the IL-17A circadian rhythm overlaid
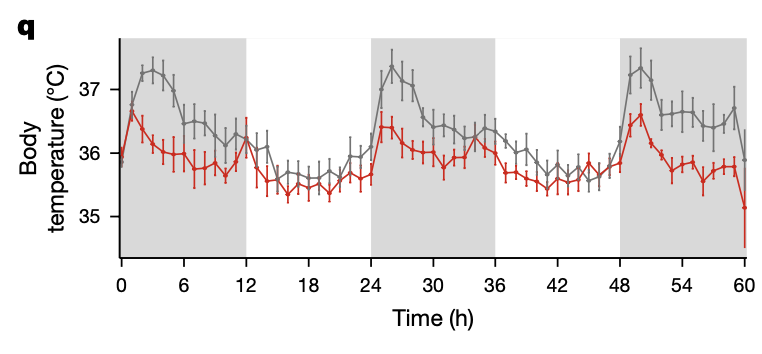
Wild-type (grey) and Il17a/f −/− body temperature.
Circadian rhythmicity analysis. White and grey panels represent light and dark periods, respectively. Data are representative of one independent experiment